

Metal fluorides are also very toxic while organic fluorides are often quite harmless. Even exposure to low concentrations causes lung and eye irritation. Additional Notes: Fluorine gas is highly toxic and corrosive.Also in toothpaste as sodium fluoride (NaF) and stannous fluoride (SnF 2) also in Teflon. Used in refrigerants and other chloro fluorocarbons. Uses of Fluorine: Combines more readily than any other element.
Primary mining areas are Canada, USA, UK, Russia, Mexico and Italy. Around 2,400 tons of fluorine gas and 4,700,000 tons of fluorite are produced each year. Sources of Fluorine: Found in the minerals fluorite (CaF 2) and cryolite (Na 2AlF 6).70kg human: 2.6 g Who / Where / When / How Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. Target Organs: Eyes, skin, respiratory system, liver, kidneys.
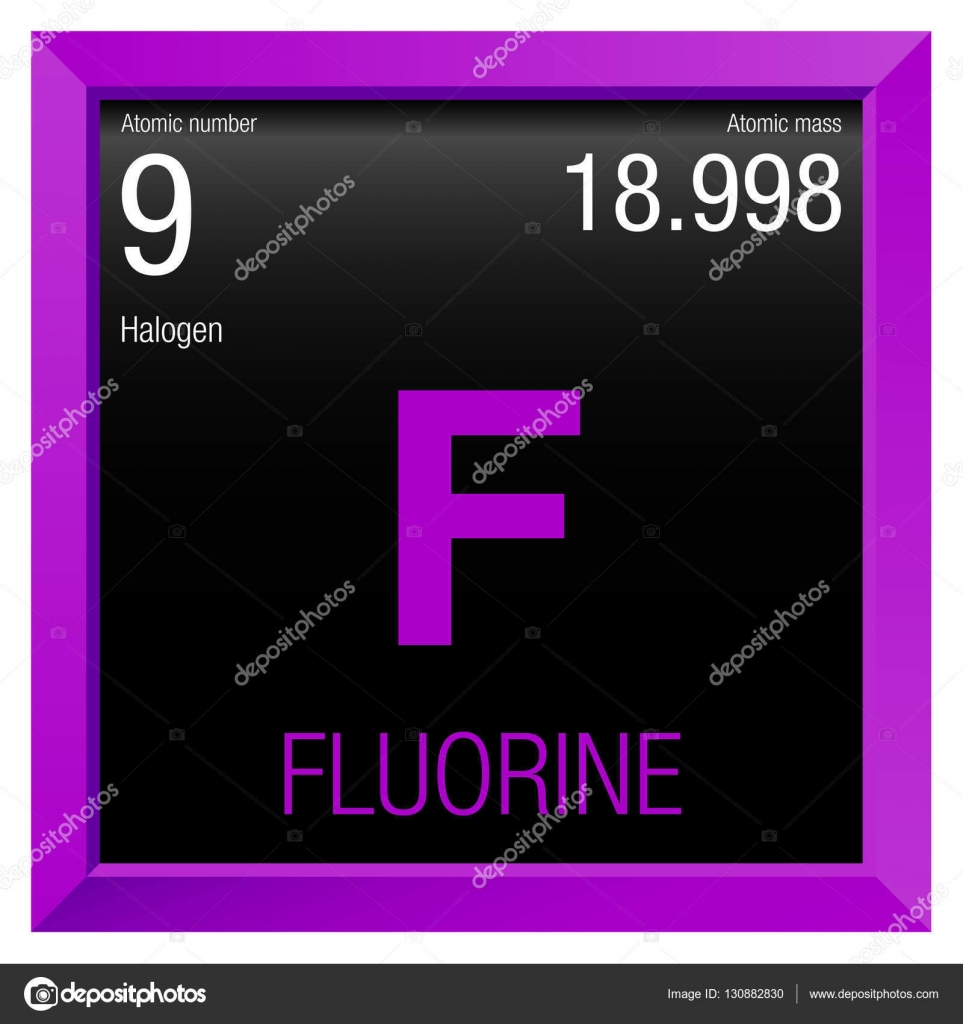
#Number 9 on periodic table skin

Swedish: Fluor Atomic Structure of Fluorine.Series: Halogens Fluorine's Name in Other Languages.Common Chemical Compounds of Fluorine Overview of Fluorine.In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies. Common chemical compounds are also provided for many elements. Skip to site menu on this page Periodic Table of Elements Element Fluorine - FĬomprehensive data on the chemical element Fluorine is provided on this page including scores of properties, element names in many languages, most known nuclides of Fluorine.


 0 kommentar(er)
0 kommentar(er)
